Apr 06, 2023
No image
Service categories
Service Lines
Big Data
Domain focus
Healthcare
Programming language
Python
Frameworks
Flask
CMS solutions
WordPress
Subcategories
Big Data
Text Analytics
Challenge
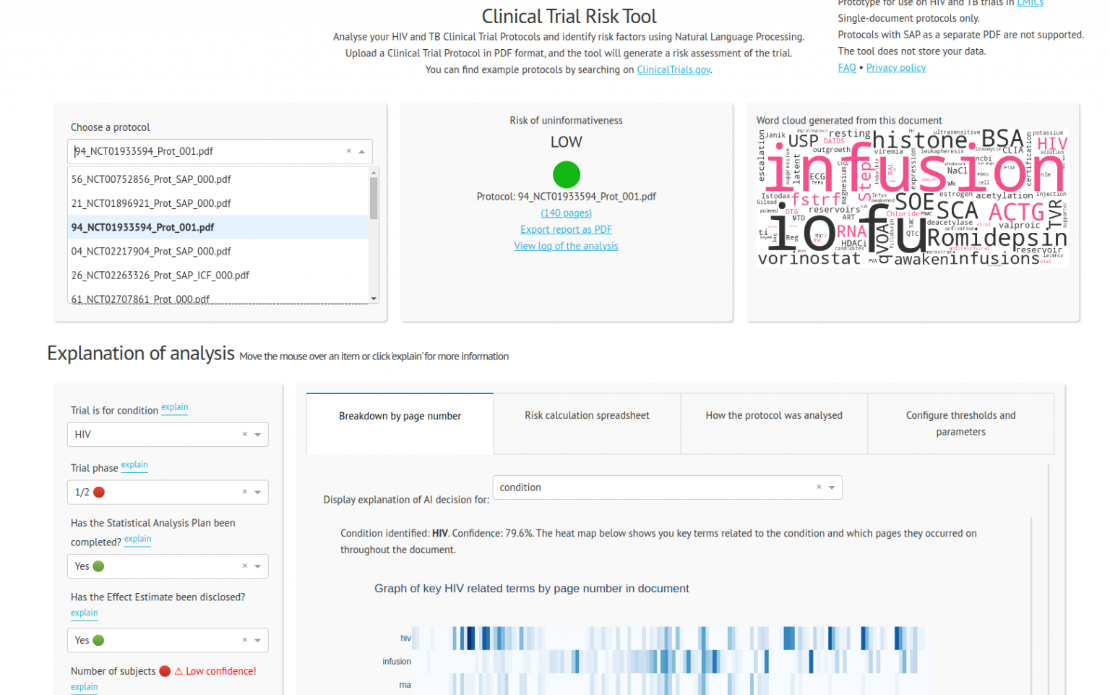
We were contacted by a client in the pharmaceutical space who wanted a tool to assist reviewers in quantifying the risk of a clinical trial protocol. A protocol is a PDF document, typically up to 200 pages long, and contains a complete description of the plan of a trial: where it will take place, how many subjects will be recruited (the sample size), which interventions are to be tested, and how the statistical analysis is to be conducted.
We were contacted by a client in the pharmaceutical space who wanted a tool to assist reviewers in quantifying the risk of a clinical trial protocol. A protocol is a PDF document, typically up to 200 pages long, and contains a complete description of the plan of a trial: where it will take place, how many subjects will be recruited (the sample size), which interventions are to be tested, and how the statistical analysis is to be conducted.
Solution
Over a period of more than a year, we experimented with an ensemble of machine learning and rule-based models to extract features such as the pathology, phase, sample size, number of countries, number of arms, presence or absence of a statistical analysis plan, effect size, and whether simulation had been used to determine the sample size. These parameters were put into a simple linear risk model and the tool generates a PDF or Excel report which can be shared within the organisation.
We deployed the tool to the internet at https://app.clinicaltrialrisk.org and open-sourced the code under MIT licence.
Over a period of more than a year, we experimented with an ensemble of machine learning and rule-based models to extract features such as the pathology, phase, sample size, number of countries, number of arms, presence or absence of a statistical analysis plan, effect size, and whether simulation had been used to determine the sample size. These parameters were put into a simple linear risk model and the tool generates a PDF or Excel report which can be shared within the organisation.
We deployed the tool to the internet at https://app.clinicaltrialrisk.org and open-sourced the code under MIT licence.
Results
The tool has enabled the funding organisation to assess incoming trials for rapid triage. It has also helped professionals worldwide to make a rough risk assessment of their trials before submitting them for funding.
The tool has enabled the funding organisation to assess incoming trials for rapid triage. It has also helped professionals worldwide to make a rough risk assessment of their trials before submitting them for funding.
